Background. Patients with newly diagnosed multiple myeloma (NDMM) have a nine-fold increased risk of developing venous thromboembolism (VTE). Despite the availability of the IMWG and NCCN VTE prophylaxis guidelines and updated risk stratification models such as SAVED and IMPEDE, the optimal thromboprophylaxis strategy is unknown, especially in the age of modern daratumumab-containing regimens. To continue highlighting the importance of appropriate thromboprophylaxis for patients with NDMM, we performed a post-hoc analysis of the randomized phase 3 MAIA trial to identify rates of venous and arterial thromboembolism.
Methods. This retrospective study, carried out under YODA Project #2021-4663, used data from the randomized phase 3 MAIA trial that investigated lenalidomide and dexamethasone (Rd) with or without daratumumab (DRd) in 733 transplant ineligible (TIE) patients obtained from the Yale University Open Data Access (YODA) Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C.. Thrombotic events (TE), including both venous and arterial events (V/ATE) were examined with VTE events including deep vein thrombosis (DVT), pulmonary embolism (PE), and superficial thrombophlebitis (SVT). Arterial events (ATE) included cerebrovascular accidents (CVA) defined as hemorrhagic, ischemic, or verebrobasilar strokes. The incidence and time to event of V/ATE were calculated and graded using the CTCAE v4, with Grade 1 representing nonactionable venous thrombosis, Grades 2-4 representing TEs requiring increasing higher levels of medical intervention, and Grade 5 indicating death was a result of the TE. Rates and time to events were compared between the DRd and Rd arms.
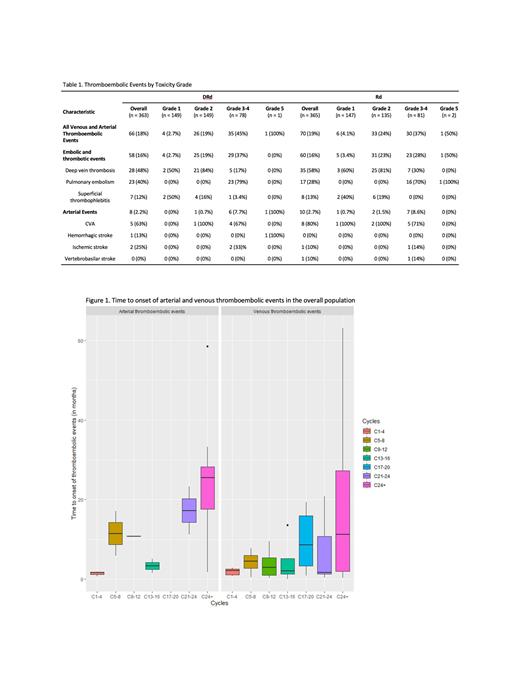
Results. Of the included patients, median age was 72 years (range 64-72) and 48% were female. There was no signficant difference between baseline characteristics of sex, race, myeloma subtype, presence of high-risk cytogenetics, or ISS staging between patients that experienced a V/ATE event and those that did not. All grade V/ATE events were 18% (66/363) and 19% (70/365) in the DRd and Rd arms, respectively. Of these, most were VTE events in both arms (16%), with G2-4 actionable VTE event rate of 14.9% vs 14.8% for the DRd and Rd arms, respectively. G5 VTE events were 0.2% in both arms (no G5 ATEs). ATE were 2.2% and 2.7% in the DRd and Rd group, respectively, overall constituting a minority of events. The overall PE rate was higher in the DRd than Rd arm, 6.3% vs 4.7%, DVT was more frequent in the Rd arm, 9.6% vs 7.7%, and rates of SVT rates were similar between both arms at 1.9% and 2.2% for the DRd vs Rd arms, respectively (Table 1). Median time to onset of cumulative TEs was longer in DRd vs Rd arm, 7.15 (0.27 - 63.0) vs 4.65 (0.03 - 58.0) months, respectively. Among these, median time to onset of VTE was similar among the groups [6.57 (0.27 - 63.0) vs 4.48 (0.03 - 52.9) months, respectively] while the median time to onset of ATEs was longer in DRd vs Rd arm, 19.8 (1.70 - 26.6) vs 8.3 (0.67 - 58.5) months. The incidence of VTE events persisted past 24 months and incidence of ATE events increased over time (Figure 1). Analysis regarding prophylactic anticoagulation use and rates of TE based on prophylactic regimen are ongoing.
Conclusion. This retrospective study reveals the highest reported rates of VTE in any modern therapy dataset. In patients with transplant ineligible NDMM, we show that the TE risk persists past 24 months of treatment in patients on DRd or Rd therapy. Similar to observations in the GRIFFIN trial, the addition of daratumumab did not increase the risk of TE. Our report highlights the critical need for further investigation of optimal prophylactic strategies and their duration for patients with NDMM.
Disclosures
Wagner:Abbvie Inc.: Other: Partner is employed as a medical science liasion. Savona:Incyte Corporation: Research Funding; Astex Pharmaceuticals: Research Funding; ALX Oncology: Research Funding; Boehringer Ingelheim: Patents & Royalties; TG Therapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda Pharmaceutical Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology, Inc.: Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics Inc.: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Geron Corporation: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma Corp.: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; AbbVie Inc.: Membership on an entity's Board of Directors or advisory committees. Julian:Pfizer: Membership on an entity's Board of Directors or advisory committees; BioLine Rx: Membership on an entity's Board of Directors or advisory committees. Godara:Janssen: Honoraria. Baljevic:Parexel: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen Biotech: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Cardinal Health: Consultancy. Sborov:BinayTara Foundation: Other: Support for attending meetings and/or travel; Arcellx: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Abbvie: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Other: Payment for an educational seminar.